Abstract
Introduction
B-acute lymphoblastic leukemia (B-ALL) is the most common cancer of childhood. Early response to induction chemotherapy is one of the important prognostic factors in B-ALL. However, the analytic sensitivity for flow cytometry (FC) is only 10 -4. The feasibility of using next-generation sequencing (NGS) of immunoglobulin for the determination of minimal residual disease (MRD) in B-ALL has been demonstrated. This study aimed to investigate the performance of NGS techniques measuring immunoglobulin heavy chain (IgH)-variable, diversity, and joining (V[D]J) clonal rearrangements compared with FC in detecting MRD for children with B-ALL and to predict the clinical outcome of B-ALL patients.
Methods
Newly diagnosed younger than 18 years old B-ALL patients who received the treatment strategy of South China children's leukemia Group (SCCLG)-ALL 2016 were recruited. DNA extracted from bone marrow cells at all available time points for each patient was submitted to Simcere diagnostics for sequencing using Illumina NovaSeq platform. We performed IgH V(D)J NGS and FCM on the bone marrow serially obtained at diagnosis (D0), 15 days at induction therapy (D15), 33 days at induction therapy (D33) and then at the end of induction therapy (EOI). We defined MRD positive (MRD +) by IgH V(D)J NGS and FCM as more than 1 blast cell among 10 4 and 10 6 bone marrow cells, respectively. The sensitivity of MRD detection by IgH V(D)J NGS and FCM, and the association of MRD status with clinicopathological characteristics were investigated. Statistical analysis was performed through SPSS Statistics 22. Enumeration data and correlation between MRD data and clinicopathological characteristics were compared by Chi-square test or Fisher's exact test. This trial was registered at www.clinicaltrials.gov as # NCT04977895.
Results
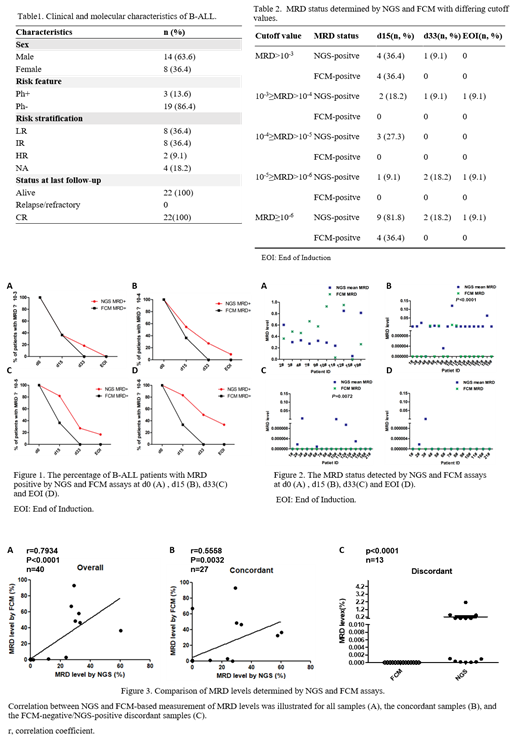
As of July 27, 2021, 22 patients (median age, 4.5 years; range, 3.0-7.3) were enrolled in the study. Three patients (13.6%) had a t (9;22) translocation consistent with Philadelphia chromosome positive disease. According to risk stratifications, 8 (36.4%), 8 (36.4%), and 2 (9.1%) patients were classified as low risk (LR), intermediate risk (IR), and high risk (HR) groups, respectively. The remaining 4 patients are still under treatment and have not been classified. We identified leukemic IgH clones in 100% of the diagnostic samples and 68.2% (15/22) of the patients were polyclonal. In 11 patients whose samples of all the four timepoints (D0, D15, D33, EOI) have been tested in parallel by FCM and IgH V(D)J NGS, the frequencies of patients with MRD + were 30.4% vs. 90.9% at D15 (P<0.05) by FCM and IgH V(D)J NGS. IgH V(D)J NGS MRD monitoring could identify MRD + patients with frequency of 45.5% and 18.2% among patients achieved MRD negativity by FCM at D33 (P<0.05) and EOI (P = 0.46). With an MRD detection limit of 10 -6, 90.9% (10/11), 36.4% (4/11) and 18.2% (2/11) patients were MRD negative by FCM but positive by the NGS test at D15, D33 and EOI, respectively. This suggested that the sensitivity of IgH V(D)J NGS was significantly higher than that of FCM. Correlation of the measured MRD between the two methods in the entire cohort (r = 0.7934, P < 0.0001) as well as in the concordant cases (r = 0.5558, P = 0.0032) was very high. There was a high discordant rate with NGS identifying more patients MRD + at this threshold. Furthermore, NGS MRD was positive but the FCM MRD was negative in 13 samples (P < 0.0001). In addition, positive MRD status of D33 by NGS was significantly associated with the age of B-ALL patients, patients under 6 years more frequently harbored detectable MRD compared with those ≥ 6 years old (87.5% vs. 11.1%, P < 0.01). There was no patient relapsed after a medium follow-up of 10.5 months.
Conclusions
We demonstrated the higher sensitivity of IgH-V(D)J NGS in MRD detection of B-ALL, which implies that NGS MRD monitoring could be helpful for more accurate risk stratifications and more precise treatment according to risk stratifications. Further study with a larger sample size and a longer follow-up period is need.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal